Abstract
Background
Umbilical cord blood (UCB) is an important alternative graft source for allogeneic hematopoietic cell transplantation (HCT) for patients without a HLA - matched donor. However, platelet recovery is significantly hampered following UCB transplantation, and patients remain platelet transfusion-dependent for prolonged periods of time. In the largest registry study to date, median time to platelet recovery over 20K/µL was over 70 days, and at 6 months only 50% of the patients had platelet engraftment. We hypothesized that eltrombopag (Revolade, GSK/Novartis), a thrombopoietin-receptor agonist, would enhance platelet engraftment after UCB transplantation.
Methods
This was a pilot multicenter open label single arm phase 2a study, in both adults and children (NCT01757145, NCT01940562). Oral eltrombopag was given from day +1 until platelet count exceeded 50K/µL for 14 consecutive days without platelet transfusion. Starting dose was 100 mg/d for adults and children weighing >40 kg and 50 mg/d for children weighing 20-40 kg. Based upon platelet response dosage was modified, with a maximal dose of 300mg/d for adults and 200mg/d for children. Study endpoints included safety, time to non-transfused platelet counts >20K/µL and >50K/µL, and platelet engraftment rate by day +50.
Results
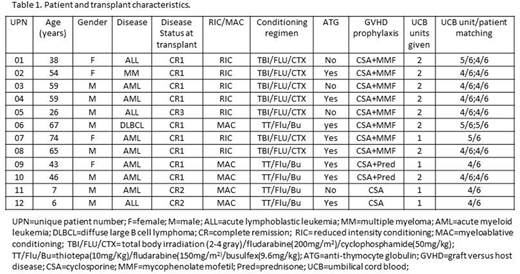
Between February 2013 and July 2016, 12 patients with hematological malignancies in complete remission were enrolled. The study cohort included 10 adults and 2 children, with a median age at transplantation of 50 (range 6-74) years (Table 1). Conditioning regimen was reduced-intensity in 7 patients and myeloablative in the remainder. Four patients received a single UCB unit, and 8 patients were given 2 units. The median total collected total nucleated cells (TNCs) *10^7/kg was 5.9 (range 2.7-8.6), and the median total collected CD34+ cells *10^5/kg was 3.1 (range 1.7-7.8).
Patients received eltrombopag for a median of 76 days (range 15-175). Overall, eltrombopag was well tolerated even at maximal doses, and there was no need for dose reduction due to toxicity. No clinically significant side effects related to the study drug were reported. Of note, two patients developed mild, asymptomatic and transient indirect hyperbilirubinemia. No patient developed sinusoidal obstructive syndrome. No patient developed a thrombotic event while receiving eltrombopag. There was one case of lower extremity deep vein thrombosis, 6 months post-transplant (#03). No significant bone marrow fibrosis was documented even after prolonged eltrombopag administration.
One patient (#07) died due to fungal respiratory infection on day +15 prior to engraftment. One patient (#01) had primary graft failure and autologous reconstitution, and is still alive in complete remission (CR) 5 years post-transplant. Among the 10 evaluable patients, median time to neutrophil engraftment was 23 (range 16-40) days. Median time to platelets>20K/µL and platelets>50K/µL was 55 (range 25-199) and 66 (range 31-230) days, respectively. Median time to RBC transfusion independence was 39 (range 13-157) days.
Overall, 4 patients developed grade 2 (n=2) or grade 3 (n=2) acute graft versus host disease (GVHD), and 4 patients developed chronic GVHD (mild, n=3; moderate, n=1). There was no death attributable to GVHD.
The median follow up time was 20 months (range 15 days - 61 months). At last follow up, 7 patients were alive, all still in CR at a median of 3.5 years post-transplant (range 1-5 years). Two patients died of relapse of their primary disease (#05, 7 months after transplant; #02, 29 months after transplant), for both these patients the UCB transplantation was the second transplant. Three patients died of infectious complications (#07, early death due to fungal respiratory infection on day +15; #08, pneumonia on day +157; #06 sepsis on day +153).
Conclusions
In the present study, eltrombopag was safe in the context of UCB transplantation. With the limitation of our small sample size, the use of eltrombopag was associated with enhanced platelet recovery time compared to published data. Further prospective studies are warranted to confirm these results.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal